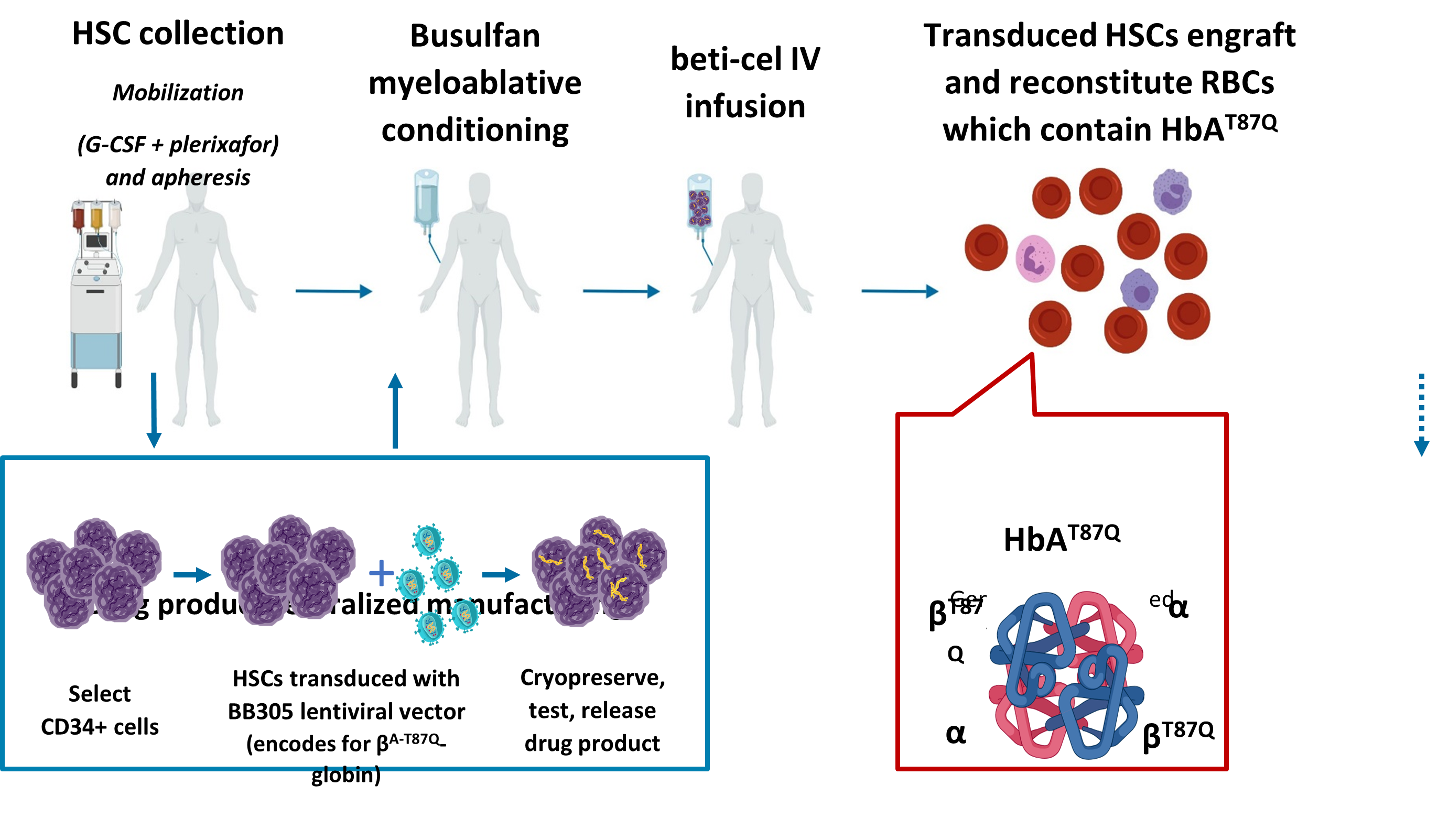
Beti-cel (ZYNTEGLOÒ) is a one-time gene therapy to treat transfusion-dependent β-thalassemia (also known as β-thalassemia major or Cooley’s Anemia). β-thalassemia is caused by a change in the β-globin gene, which causes the body to produce reduced or no β-globin. Beti-cel drug product is made specifically for each patient, using the patient’s own blood stem cells into which a functional copy of the beta-globin gene has been inserted in hematopoietic stem cells cells. This insertion and repopulation of gene therapy modified stem cells may be sufficient to stop receiving regular transfusions in the majority of those who receive the treatment. bluebird bio therapies that are currently approved for use by the U.S. Food and Drug Administration (FDA) are only available in the U.S. through a network of Qualified Treatment Centers (QTCs) —specialized hospitals that are trained to administer gene therapy. The program at UCSF Benioff Children’s Hospital, Oakland was one of the first in the US to roll out this new treatment. Referrals are being accepted.
More more information, please contact the Bone& Marrow Transplant Program at 510-428-3885 ext. 5183.